Equipment
Explore our diverse range of compounding equipment—designed to streamline workflows, automate processes, and deliver reproducible quality, all while adapting to the needs of the future.
Shop equipment
Non-sterileSterile
Find the right
pharmacy hood
Whether you're compounding sterile or non-sterile preparations, our pharmacy hoods provide the containment and control needed to support safe, effective workflows.

Achieve efficient
capsule filling
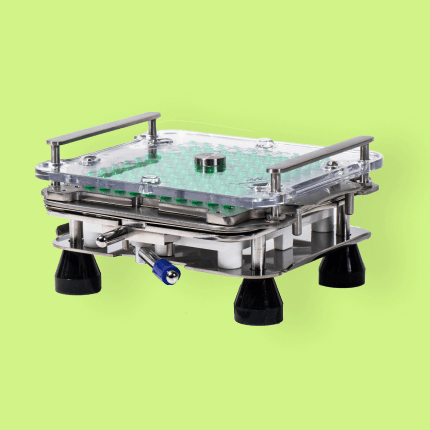
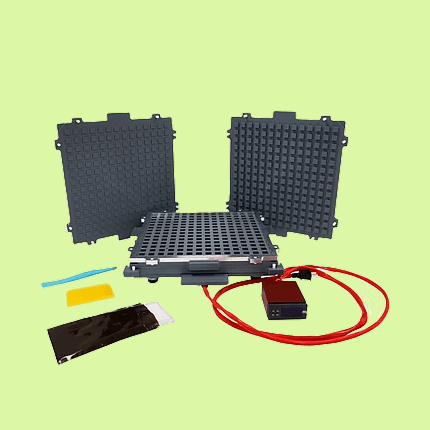
Boost productivity with a capsule machine designed to streamline your workflow, reduce preparation time, and deliver repeatable results—batch after batch.
Customer
favorites

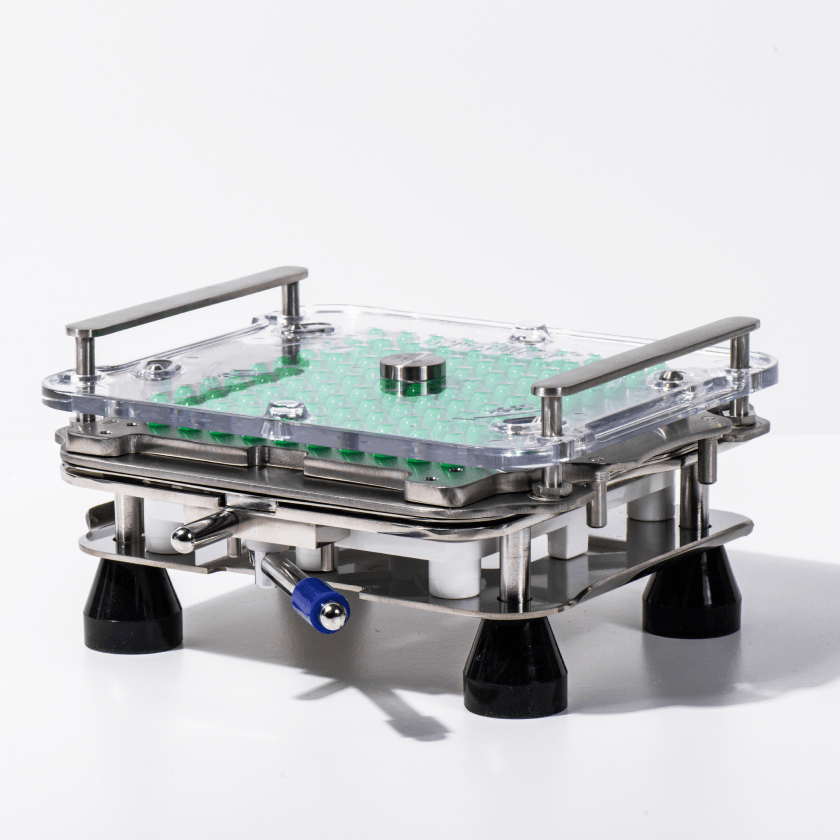
The Medisca MAZ® mixer is a versatile mixer and deaerator system perfect for creams, gels, ointments, and liquids. Its vacuum-like function removes air entrapment, combining mixing and deaeration in one step. With no need for mixing rods or blades, it achieves high-speed mixing up to 2000 rpm and reduces processing times.
Learn moreEverything you need
to equip your lab
Discover innovative solutions that streamline your compounding process and boost efficiency at every stage of your workflow.
Adhere to USP standards
Ensure your equipment purchases comply with USP regulatory standards.
Standard operating
procedures
Streamline compliance and achieve accreditation with Medisca standard operating procedures (SOPs). Downloadable, customizable, and user-friendly compounding procedures that support regulatory adherence and elevate standards of practice.
Specialized
consultations
Experience personalized one-on-one support with our expert team of consultants, drawing on more than 20+ years of compounding experience.
Need help
getting started?